Search
- Page Path
- HOME > Search
Review Article
- Calcium & bone metabolism
- Bone Loss after Solid Organ Transplantation: A Review of Organ-Specific Considerations
- Kyoung Jin Kim, Jeonghoon Ha, Sang Wan Kim, Jung-Eun Kim, Sihoon Lee, Han Seok Choi, Namki Hong, Sung Hye Kong, Seong Hee Ahn, So Young Park, Ki-Hyun Baek, on Behalf of Metabolic Bone Disease Study Group of Korean Endocrine Society
- Endocrinol Metab. 2024;39(2):267-282. Published online April 25, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1939
- 35 View
- 2 Download
-
 Abstract
Abstract
 PDF
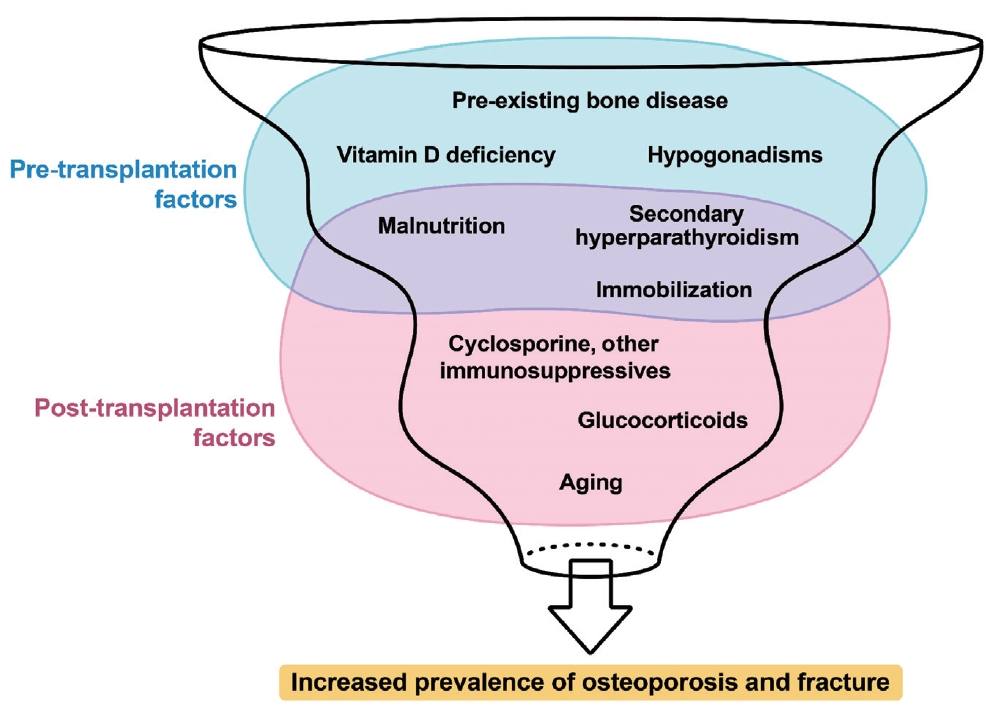
PDF - This review article investigates solid organ transplantation-induced osteoporosis, a critical yet often overlooked issue, emphasizing its significance in post-transplant care. The initial sections provide a comprehensive understanding of the prevalence and multifactorial pathogenesis of transplantation osteoporosis, including factors such as deteriorating post-transplantation health, hormonal changes, and the impact of immunosuppressive medications. Furthermore, the review is dedicated to organ-specific considerations in transplantation osteoporosis, with separate analyses for kidney, liver, heart, and lung transplantations. Each section elucidates the unique challenges and management strategies pertinent to transplantation osteoporosis in relation to each organ type, highlighting the necessity of an organ-specific approach to fully understand the diverse manifestations and implications of transplantation osteoporosis. This review underscores the importance of this topic in transplant medicine, aiming to enhance awareness and knowledge among clinicians and researchers. By comprehensively examining transplantation osteoporosis, this study contributes to the development of improved management and care strategies, ultimately leading to improved patient outcomes in this vulnerable group. This detailed review serves as an essential resource for those involved in the complex multidisciplinary care of transplant recipients.

Original Article
- Thyroid
Thyroid Cancer Screening - Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
- Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chul-Min Kim
- Endocrinol Metab. 2024;39(2):310-323. Published online April 9, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1870
- 388 View
- 11 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
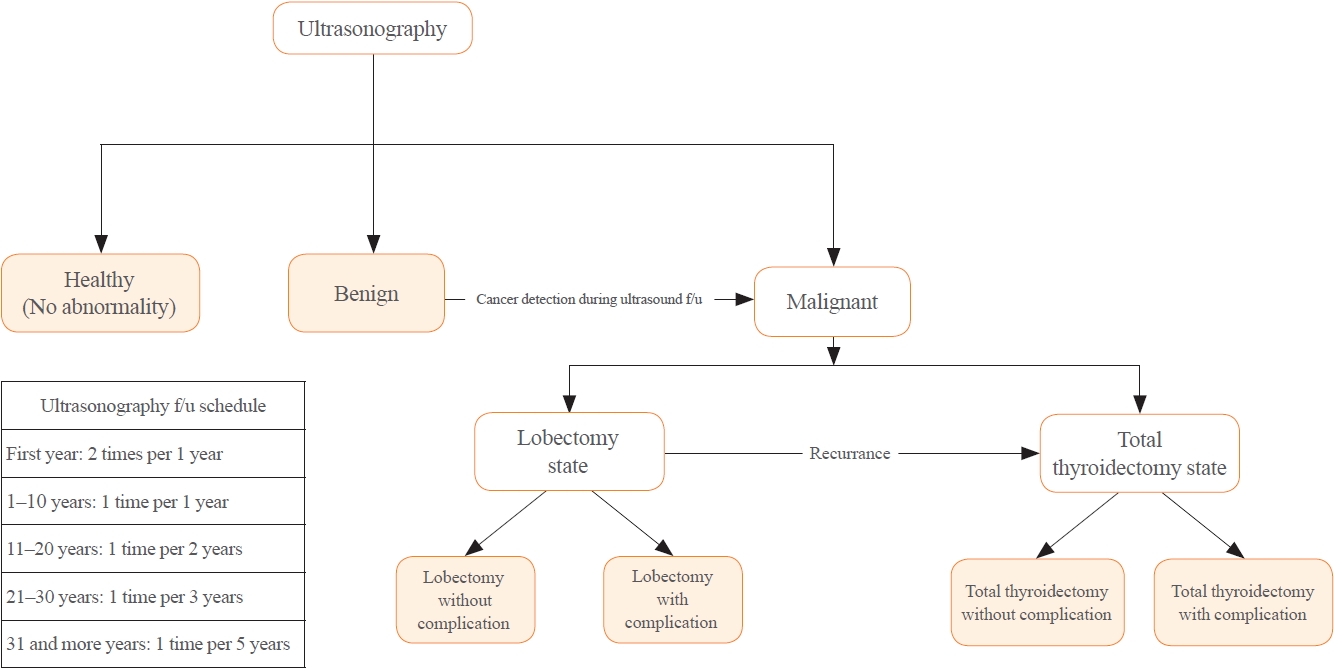
There is debate about ultrasonography screening for thyroid cancer and its cost-effectiveness. This study aimed to evaluate the cost-effectiveness of early screening (ES) versus symptomatic detection (SD) for differentiated thyroid cancer (DTC) in Korea.
Methods
A Markov decision analysis model was constructed to compare the cost-effectiveness of ES and SD. The model considered direct medical costs, health outcomes, and different diagnostic and treatment pathways. Input data were derived from literature and Korean population studies. Incremental cost-effectiveness ratio (ICER) was calculated. Willingness-to-pay (WTP) threshold was set at USD 100,000 or 20,000 per quality-adjusted life year (QALY) gained. Sensitivity analyses were conducted to address uncertainties of the model’s variables.
Results
In a base case scenario with 50 years of follow-up, ES was found to be cost-effective compared to SD, with an ICER of $2,852 per QALY. With WTP set at $100,000, in the case with follow-up less than 10 years, the SD was cost-effective. Sensitivity analysis showed that variables such as lobectomy probability, age, mortality, and utility scores significantly influenced the ICER. Despite variations in costs and other factors, all ICER values remained below the WTP threshold.
Conclusion
Findings of this study indicate that ES is a cost-effective strategy for DTC screening in the Korean medical system. Early detection and subsequent lobectomy contribute to the cost-effectiveness of ES, while SD at an advanced stage makes ES more cost-effective. Expected follow-up duration should be considered to determine an optimal strategy for DTC screening.

Special Article
- Adrenal gland
- 2023 Korean Endocrine Society Consensus Guidelines for the Diagnosis and Management of Primary Aldosteronism
- Jeonghoon Ha, Jung Hwan Park, Kyoung Jin Kim, Jung Hee Kim, Kyong Yeun Jung, Jeongmin Lee, Jong Han Choi, Seung Hun Lee, Namki Hong, Jung Soo Lim, Byung Kwan Park, Jung-Han Kim, Kyeong Cheon Jung, Jooyoung Cho, Mi-kyung Kim, Choon Hee Chung, The Committee of Clinical Practice Guideline of Korean Endocrine Society, The Korean Adrenal Study Group of Korean Endocrine Society
- Endocrinol Metab. 2023;38(6):597-618. Published online October 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1789
- 3,456 View
- 482 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
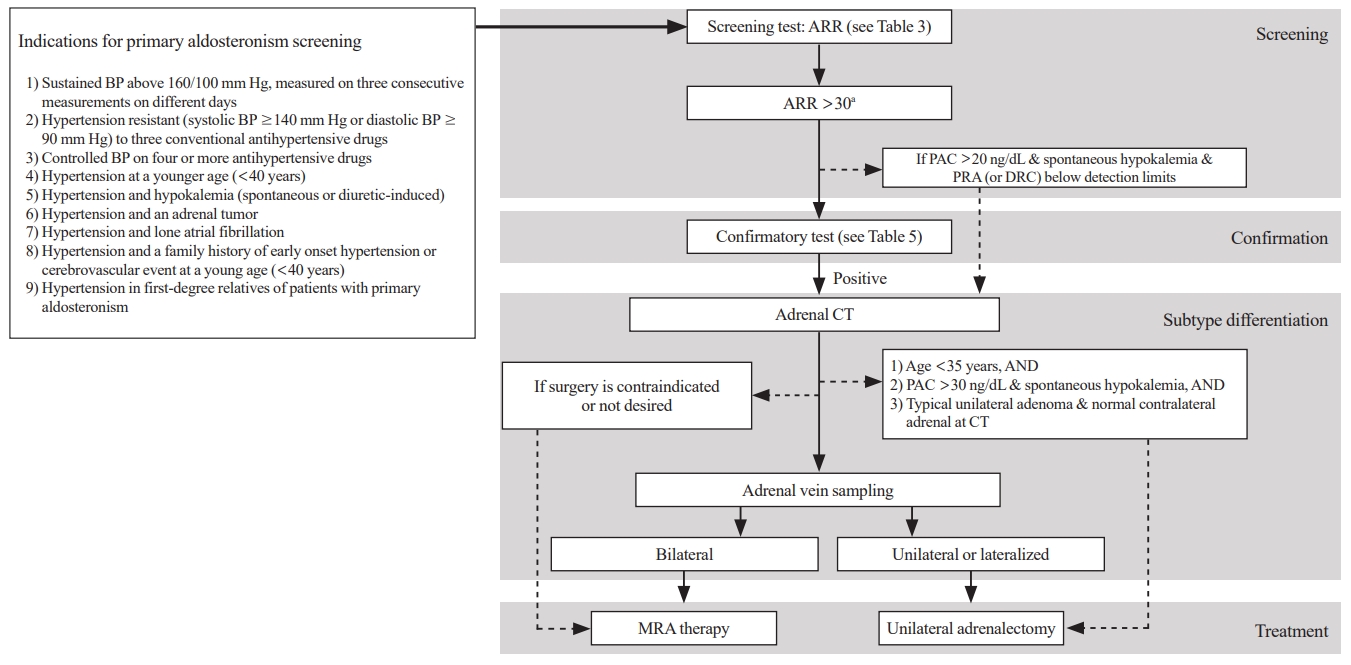
ePub - Primary aldosteronism (PA) is a common, yet underdiagnosed cause of secondary hypertension. It is characterized by an overproduction of aldosterone, leading to hypertension and/or hypokalemia. Despite affecting between 5.9% and 34% of patients with hypertension, PA is frequently missed due to a lack of clinical awareness and systematic screening, which can result in significant cardiovascular complications. To address this, medical societies have developed clinical practice guidelines to improve the management of hypertension and PA. The Korean Endocrine Society, drawing on a wealth of research, has formulated new guidelines for PA. A task force has been established to prepare PA guidelines, which encompass epidemiology, pathophysiology, clinical presentation, diagnosis, treatment, and follow-up care. The Korean clinical guidelines for PA aim to deliver an evidence-based protocol for PA diagnosis, treatment, and patient monitoring. These guidelines are anticipated to ease the burden of this potentially curable condition.
-
Citations
Citations to this article as recorded by- Correlation of Histopathologic Subtypes of Primary Aldosteronism with Clinical Phenotypes and Postsurgical Outcomes
Chang Ho Ahn, You-Bin Lee, Jae Hyeon Kim, Young Lyun Oh, Jung Hee Kim, Kyeong Cheon Jung
The Journal of Clinical Endocrinology & Metabolism.2023;[Epub] CrossRef
- Correlation of Histopathologic Subtypes of Primary Aldosteronism with Clinical Phenotypes and Postsurgical Outcomes

Original Articles
- Thyroid
- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
- Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
- Endocrinol Metab. 2023;38(3):338-346. Published online June 9, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1664
- 1,733 View
- 102 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
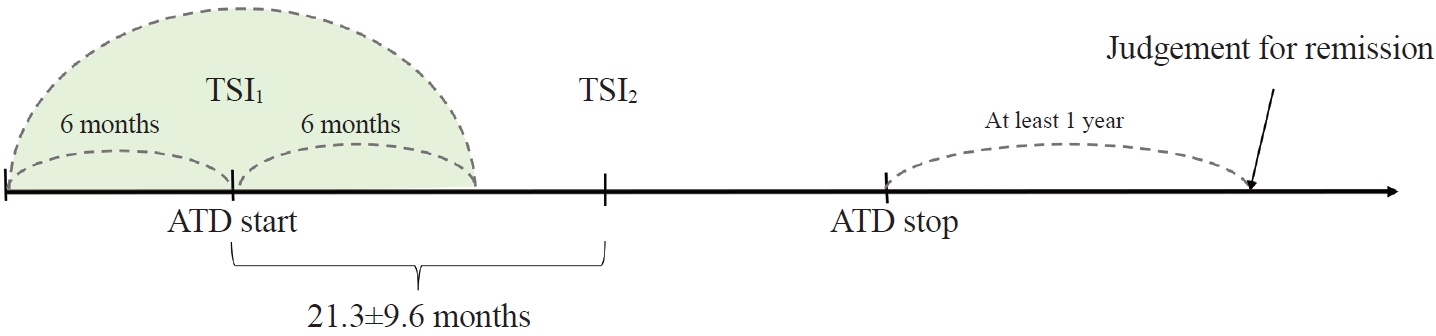
To determine whether baseline thyroid-stimulating immunoglobulin (TSI) bioassay or its early response upon treatment with an anti-thyroid drug (ATD) can predict prognosis of Graves’ disease (GD) in real-world practice.
Methods
This retrospective study enrolled GD patients who had previous ATD treatment with TSI bioassay checked at baseline and at follow-up from April 2010 to November 2019 in one referral hospital. The study population were divided into two groups: patients who experienced relapse or continued ATD (relapse/persistence), and patients who experienced no relapse after ATD discontinuation (remission). The slope and area under the curve at 1st year (AUC1yr) of thyroid-stimulating hormone receptor antibodies including TSI bioassay and thyrotropin-binding inhibitory immunoglobulin (TBII) were calculated as differences between baseline and second values divided by time duration (year).
Results
Among enrolled 156 study subjects, 74 (47.4%) had relapse/persistence. Baseline TSI bioassay values did not show significant differences between the two groups. However, the relapse/persistence group showed less decremental TSI bioassay in response to ATD than the remission group (–84.7 [TSI slope, –198.2 to 8.2] vs. –120.1 [TSI slope, –204.4 to –45.9], P=0.026), whereas the TBII slope was not significantly different between the two groups. The relapse/persistence group showed higher AUC1yr of TSI bioassay and TBII in the 1st year during ATD treatment than the remission group (AUC1yr for TSI bioassay, P=0.0125; AUC1yr for TBII,P =0.001).
Conclusion
Early changes in TSI bioassay can better predict prognosis of GD than TBII. Measurement of TSI bioassay at beginning and follow-up could help predict GD prognosis. -
Citations
Citations to this article as recorded by- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study
Xinpan Wang, Tiantian Li, Yue Li, Qiuyi Wang, Yun Cai, Zhixiao Wang, Yun Shi, Tao Yang, Xuqin Zheng
Journal of Translational Medicine.2024;[Epub] CrossRef - Long-term effect of thyrotropin-binding inhibitor immunoglobulin on atrial fibrillation in euthyroid patients
Jung-Chi Hsu, Kang-Chih Fan, Ting-Chuan Wang, Shu-Lin Chuang, Ying-Ting Chao, Ting-Tse Lin, Kuan-Chih Huang, Lian-Yu Lin, Lung-Chun Lin
Endocrine Practice.2024;[Epub] CrossRef
- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study

- Calcium & bone metabolism
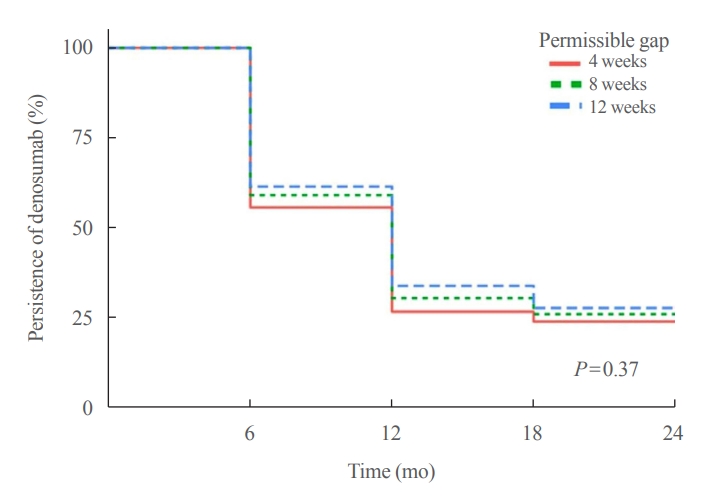
- Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
- Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
- Endocrinol Metab. 2023;38(2):260-268. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1663
- 1,754 View
- 109 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Persistence with denosumab in male patients has not been adequately investigated, although poor denosumab persistence is associated with a significant risk of rebound vertebral fractures.
Methods
We retrospectively evaluated 294 Korean male osteoporosis patients treated with denosumab at three medical centers and examined their persistence with four doses of denosumab injection over 24 months of treatment. Persistence was defined as the extent to which a patient adhered to denosumab treatment in terms of the prescribed interval and dose, with a permissible gap of 8 weeks. For patients who missed their scheduled treatment appointment(s) during the follow-up period (i.e., no-shows), Cox proportional regression analysis was conducted to explore the factors associated with poor adherence. Several factors were considered, such as age, prior anti-osteoporotic drug use, the treatment provider’s medical specialty, the proximity to the medical center, and financial burdens of treatment.
Results
Out of 294 male patients, 77 (26.2%) completed all four sequential rounds of the denosumab treatment. Out of 217 patients who did not complete the denosumab treatment, 138 (63.6%) missed the scheduled treatment(s). Missing treatment was significantly associated with age (odds ratio [OR], 1.03), prior bisphosphonate use (OR, 0.76), and prescription by non-endocrinologists (OR, 2.24). Denosumab was stopped in 44 (20.3%) patients due to medical errors, in 24 (11.1%) patients due to a T-score improvement over –2.5, and in five (2.3%) patients due to expected dental procedures.
Conclusion
Our study showed that only one-fourth of Korean male osteoporosis patients were fully adherent to 24 months of denosumab treatment. -
Citations
Citations to this article as recorded by- Denosumab
Reactions Weekly.2023; 1963(1): 206. CrossRef
- Denosumab

Special Article
- Miscellaneous
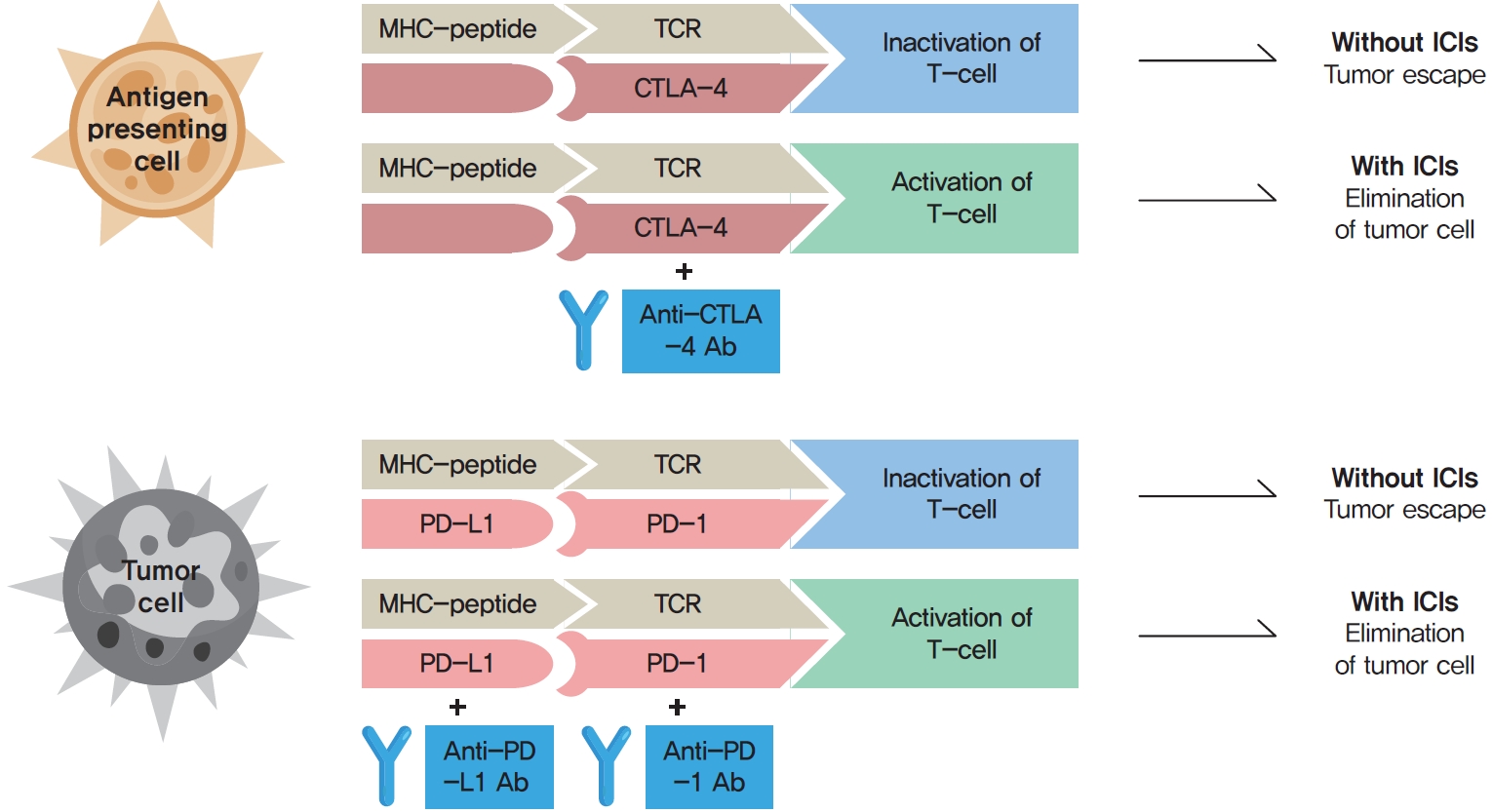
- Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
- Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim, The Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2022;37(6):839-850. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1627
- 3,488 View
- 321 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Immune checkpoint inhibitors (ICIs) including an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, anti-programmed cell death protein 1 (PD-1) inhibitors, and anti-PD-ligand 1 inhibitors are representative therapeutics for various malignancies. In oncology, the application of ICIs is currently expanding to a wider range of malignancies due to their remarkable clinical outcomes. ICIs target immune checkpoints which suppress the activity of T-cells that are specific for tumor antigens, thereby allowing tumor cells to escape the immune response. However, immune checkpoints also play a crucial role in preventing autoimmune reactions. Therefore, ICIs targeting immune checkpoints can trigger various immune-related adverse events (irAEs), especially in endocrine organs. Considering the endocrine organs that are frequently involved, irAEs associated endocrinopathies are frequently life-threatening and have unfavorable clinical implications for patients. However, there are very limited data from large clinical trials that would inform the development of clinical guidelines for patients with irAEs associated endocrinopathies. Considering the current clinical situation, in which the scope and scale of the application of ICIs are increasing, position statements from clinical specialists play an essential role in providing the appropriate recommendations based on both medical evidence and clinical experience. As endocrinologists, we would like to present precautions and recommendations for the management of immune-related endocrine disorders, especially those involving the adrenal, thyroid, and pituitary glands caused by ICIs.
-
Citations
Citations to this article as recorded by- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population
Ryo Fujiwara, Takeshi yuasa, kenichi kobayashi, tetsuya yoshida, susumu kageyama
Expert Review of Anticancer Therapy.2023; 23(5): 461. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef
- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population

Response
- Diabetes, Obesity and Metabolism
- Characteristics of Glycemic Control and Long-Term Complications in Patients with Young-Onset Type 2 Diabetes (Endocrinol Metab 2022;37:641-51, Han-sang Baek et al.)
- Han-sang Baek, Ji-Yeon Park, Jin Yu, Joonyub Lee, Yeoree Yang, Jeonghoon Ha, Seung Hwan Lee, Jae Hyoung Cho, Dong-Jun Lim, Hun-Sung Kim
- Endocrinol Metab. 2022;37(6):945-946. Published online December 2, 2022
- DOI: https://doi.org/10.3803/EnM.2022.602
- [Original]
- 1,733 View
- 165 Download

Editorial
- Calcium & Bone Metabolism
- A Meaningful Journey to Predict Fractures with Deep Learning
- Jeonghoon Ha
- Endocrinol Metab. 2022;37(4):617-619. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.403
- 1,383 View
- 87 Download

Original Articles
- Diabetes, Obesity and Metabolism
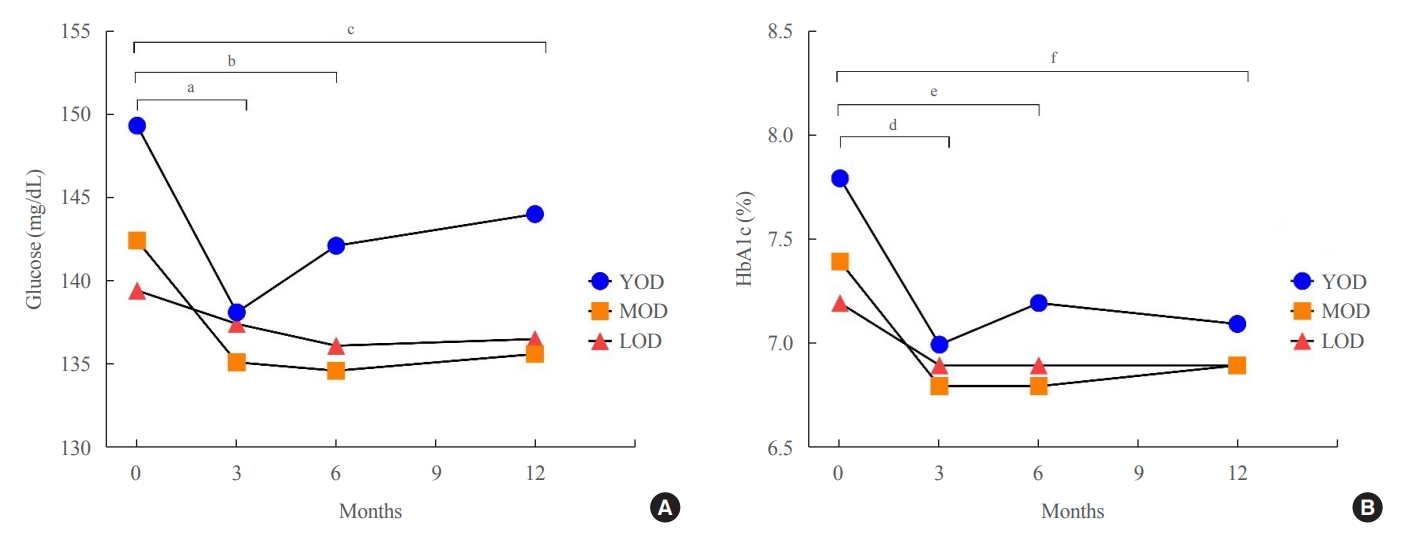
- Characteristics of Glycemic Control and Long-Term Complications in Patients with Young-Onset Type 2 Diabetes
- Han-sang Baek, Ji-Yeon Park, Jin Yu, Joonyub Lee, Yeoree Yang, Jeonghoon Ha, Seung Hwan Lee, Jae Hyoung Cho, Dong-Jun Lim, Hun-Sung Kim
- Endocrinol Metab. 2022;37(4):641-651. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1501
- 6,167 View
- 166 Download
- 10 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The prevalence of young-onset diabetes (YOD) has been increasing worldwide. As the incidence of YOD increases, it is necessary to determine the characteristics of YOD and the factors that influence its development and associated complications.
Methods
In this retrospective study, we recruited patients who were diagnosed with type 2 diabetes mellitus between June 2001 and December 2021 at a tertiary hospital. The study population was categorized according to age: YOD (age <40 years), middle-age-onset diabetes (MOD, 40≤ age <65 years), and late-onset diabetes (LOD, age ≥65 years). We examined trends in glycemic control by analyzing fasting glucose levels during the first year in each age group. A Cox proportional-hazards model was used to determine the relative risk of developing complications according to glycemic control trends.
Results
The fasting glucose level at the time of diagnosis was highest in the YOD group (YOD 149±65 mg/dL; MOD 143±54 mg/dL; and LOD 140±55 mg/dL; p=0.009). In the YOD group, glucose levels decreased at 3 months, but increased by 12 months. YOD patients and those with poor glycemic control in the first year were at a higher risk of developing complications, whereas the risk in patients with LOD was not statistically significant.
Conclusion
YOD patients had higher glucose levels at diagnosis, and their glycemic control was poorly maintained. As poor glycemic control can influence the development of complications, especially in young patients, intensive treatment is necessary for patients with YOD. -
Citations
Citations to this article as recorded by- Increased risk of incident mental disorders in adults with new-onset type 1 diabetes diagnosed after the age of 19: A nationwide cohort study
Seohyun Kim, Gyuri Kim, So Hyun Cho, Rosa Oh, Ji Yoon Kim, You-Bin Lee, Sang-Man Jin, Kyu Yeon Hur, Jae Hyeon Kim
Diabetes & Metabolism.2024; 50(1): 101505. CrossRef - Association between age at diagnosis of type 2 diabetes and cardiovascular morbidity and mortality risks: A nationwide population-based study
Da Hea Seo, Mina Kim, Young Ju Suh, Yongin Cho, Seong Hee Ahn, Seongbin Hong, So Hun Kim
Diabetes Research and Clinical Practice.2024; 208: 111098. CrossRef - Impact of diabetes distress on glycemic control and diabetic complications in type 2 diabetes mellitus
Hye-Sun Park, Yongin Cho, Da Hea Seo, Seong Hee Ahn, Seongbin Hong, Young Ju Suh, Suk Chon, Jeong-Taek Woo, Sei Hyun Baik, Kwan Woo Lee, So Hun Kim
Scientific Reports.2024;[Epub] CrossRef - Early onset type 2 diabetes mellitus: an update
Myrsini Strati, Melpomeni Moustaki, Theodora Psaltopoulou, Andromachi Vryonidou, Stavroula A. Paschou
Endocrine.2024;[Epub] CrossRef - Complications and Treatment of Early-Onset Type 2 Diabetes
Fahimeh Soheilipour, Naghmeh Abbasi Kasbi, Mahshid Imankhan, Delaram Eskandari
International Journal of Endocrinology and Metabolism.2023;[Epub] CrossRef - Characteristics of Glycemic Control and Long-Term Complications in Patients with Young-Onset Type 2 Diabetes (Endocrinol Metab 2022;37:641-51, Han-sang Baek et al.)
Han-sang Baek, Ji-Yeon Park, Jin Yu, Joonyub Lee, Yeoree Yang, Jeonghoon Ha, Seung Hwan Lee, Jae Hyoung Cho, Dong-Jun Lim, Hun-Sung Kim
Endocrinology and Metabolism.2022; 37(6): 945. CrossRef -
ISPAD
Clinical Practice Consensus Guidelines 2022: Management of the child, adolescent, and young adult with diabetes in limited resource settings
Anju Virmani, Stuart J. Brink, Angela Middlehurst, Fauzia Mohsin, Franco Giraudo, Archana Sarda, Sana Ajmal, Julia E. von Oettingen, Kuben Pillay, Supawadee Likitmaskul, Luis Eduardo Calliari, Maria E. Craig
Pediatric Diabetes.2022; 23(8): 1529. CrossRef - Characteristics of Glycemic Control and Long-Term Complications in Patients with Young-Onset Type 2 Diabetes (Endocrinol Metab 2022;37:641-51, Han-sang Baek et al.)
May Thu Hla Aye, Sajid Adhi Raja, Vui Heng Chong
Endocrinology and Metabolism.2022; 37(6): 943. CrossRef
- Increased risk of incident mental disorders in adults with new-onset type 1 diabetes diagnosed after the age of 19: A nationwide cohort study

- Calcium & Bone Metabolism
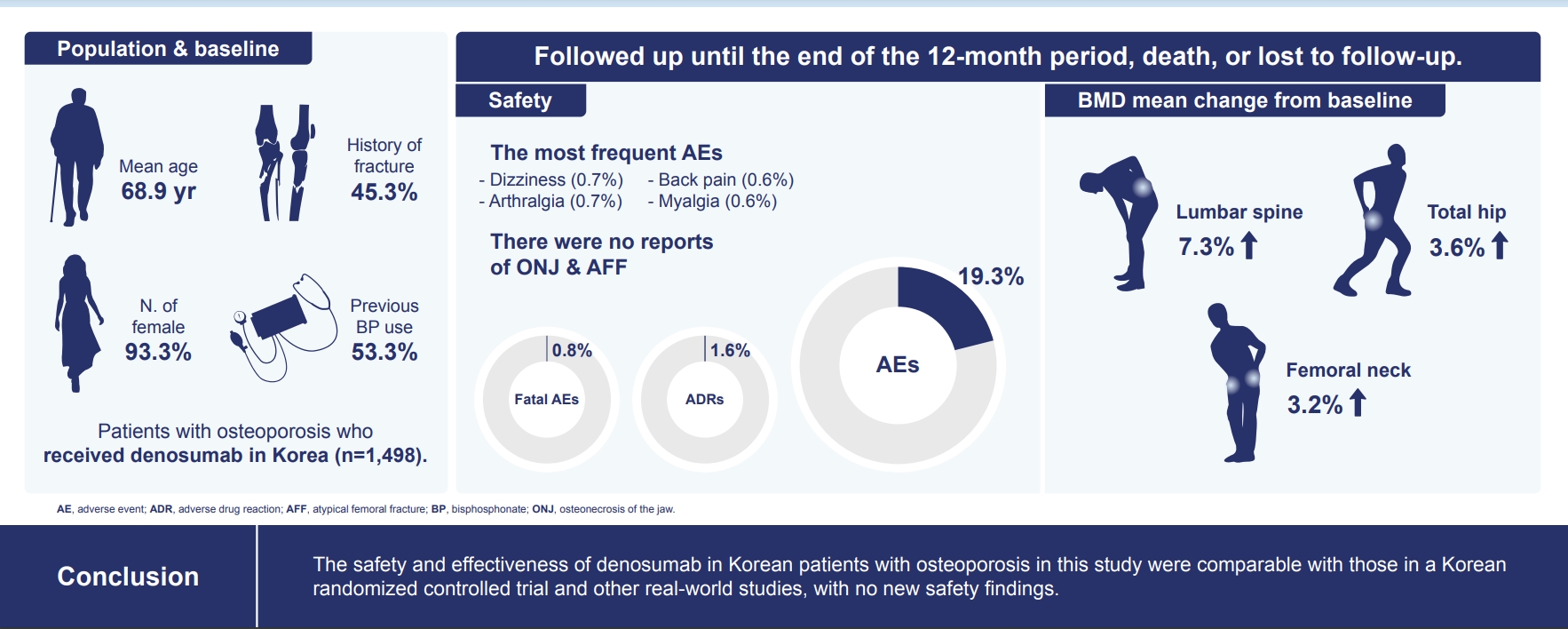
- Real-World Safety and Effectiveness of Denosumab in Patients with Osteoporosis: A Prospective, Observational Study in South Korea
- Yumie Rhee, Dong-Gune Chang, Jeonghoon Ha, Sooa Kim, Yusun Lee, Euna Jo, Jung-Min Koh
- Endocrinol Metab. 2022;37(3):497-505. Published online June 3, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1427
- 5,375 View
- 268 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The efficacy and safety of denosumab have been established in a phase 3, randomized, placebo-controlled trial in Korean postmenopausal women with osteoporosis. This postmarketing surveillance study was aimed to investigate the safety and effectiveness of denosumab in Korean real-world clinical practice.
Methods
Patients with osteoporosis who had received denosumab per the Korean approved indications in the postmarketing setting between September 2014 and September 2019 were enrolled. The primary endpoint was the incidence of adverse events (AEs) and adverse drug reactions (ADRs). The secondary endpoint was the percent change from baseline in bone mineral density (BMD) of the lumbar spine, total hip, and femoral neck.
Results
Of the 3,221 patients enrolled, 3,185 were included in the safety analysis set; 2,973 (93.3%) were female, and the mean± standard deviation (SD) age was 68.9±9.9 years. The mean±SD study period was 350.0±71.4 days. AEs, fatal AEs, and ADRs occurred in 19.3%, 0.8%, and 1.6%, respectively. The most frequent AEs, occurring in >0.5% of patients, were dizziness (0.7%), arthralgia (0.7%), back pain (0.6%), and myalgia (0.6%). Hypocalcemia occurred in 0.3% of patients. There were no cases of osteonecrosis of the jaw and atypical femoral fracture. Mean±SD percent change from baseline in BMD of the lumbar spine, total hip, and femoral neck was 7.3%±23.6%, 3.6%±31.4%, and 3.2%±10.7%, respectively.
Conclusion
The safety and effectiveness of denosumab in Korean patients with osteoporosis in this study were comparable with those in the Korean randomized controlled trial, with no new safety findings. -
Citations
Citations to this article as recorded by- Prevalence of denosumab-induced hypocalcemia: a retrospective observational study of patients routinely monitored with ionized calcium post-injection
Anna Spångeus, Johan Rydetun, Mischa Woisetschläger
Osteoporosis International.2024; 35(1): 173. CrossRef - Cost-consequence analysis of continuous denosumab therapy for osteoporosis treatment in South Korea
Seungju Cha, Minjeong Sohn, Hyowon Yang, Eric J. Yeh, Ki-Hyun Baek, Jeonghoon Ha, Hyemin Ku
BMC Musculoskeletal Disorders.2024;[Epub] CrossRef - Denosumab and the Risk of Diabetes in Patients Treated for Osteoporosis
Huei-Kai Huang, Albert Tzu-Ming Chuang, Tzu-Chi Liao, Shih-Chieh Shao, Peter Pin-Sung Liu, Yu-Kang Tu, Edward Chia-Cheng Lai
JAMA Network Open.2024; 7(2): e2354734. CrossRef - Adverse Effects of Denosumab in Kidney Transplant Recipients: A 20-Year Retrospective Single-Center Observation Study in Central Taiwan
Tsung-Yin Tsai, Zi-Hong You, Shang-Feng Tsai, Ming-Ju Wu, Tung-Min Yu, Ya-Wen Chuang, Yung-Chieh Lin, Ya-Lian Deng, Chiann-Yi Hsu, Cheng-Hsu Chen
Transplantation Proceedings.2023; 55(4): 837. CrossRef - Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
Endocrinology and Metabolism.2023; 38(2): 260. CrossRef - Effect of Denosumab on Bone Density in Postmenopausal Osteoporosis: A Comparison with and without Calcium Supplementation in Patients on Standard Diets in Korea
Chaiho Jeong, Jinyoung Kim, Jeongmin Lee, Yejee Lim, Dong-Jun Lim, Ki-Hyun Baek, Jeonghoon Ha
Journal of Clinical Medicine.2023; 12(21): 6904. CrossRef - Denosumab
Reactions Weekly.2022; 1919(1): 221. CrossRef - Denosumab, an effective osteoporosis treatment option for men
Sung Hye Kong
The Korean Journal of Internal Medicine.2022; 37(5): 947. CrossRef
- Prevalence of denosumab-induced hypocalcemia: a retrospective observational study of patients routinely monitored with ionized calcium post-injection

- Thyroid
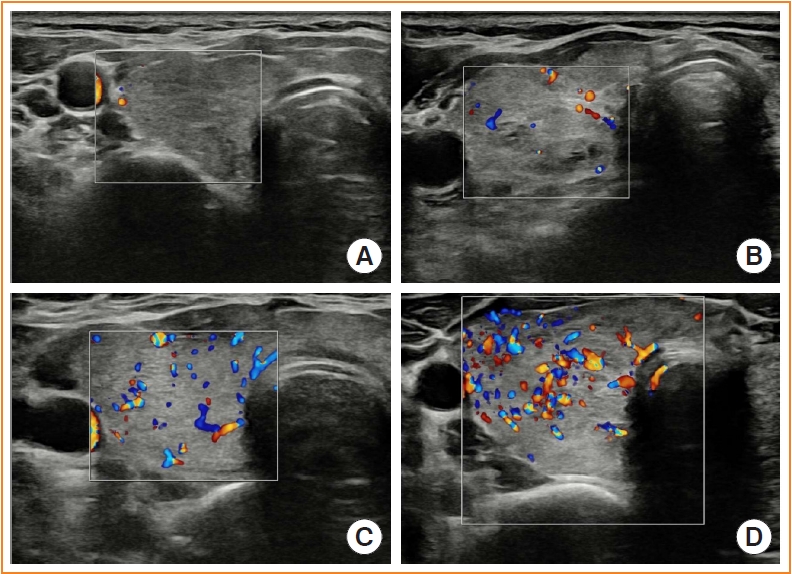
- Usefulness of Real-Time Quantitative Microvascular Ultrasonography for Differentiation of Graves’ Disease from Destructive Thyroiditis in Thyrotoxic Patients
- Han-Sang Baek, Ji-Yeon Park, Chai-Ho Jeong, Jeonghoon Ha, Moo Il Kang, Dong-Jun Lim
- Endocrinol Metab. 2022;37(2):323-332. Published online April 13, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1413
- 3,675 View
- 144 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Microvascular ultrasonography (MVUS) is a third-generation Doppler technique that was developed to increase sensitivity compared to conventional Doppler. The purpose of this study was to compare MVUS with conventional color Doppler (CD) and power Doppler (PD) imaging to distinguish Graves’ disease (GD) from destructive thyroiditis (DT).
Methods
This prospective study included 101 subjects (46 GDs, 47 DTs, and eight normal controls) from October 2020 to November 2021. All ultrasonography examinations were performed using microvascular flow technology (MV-Flow). The CD, PD, and MVUS images were semi-quantitatively graded according to blood flow patterns. On the MVUS images, vascularity indices (VIs), which were the ratio (%) of color pixels in the total grayscale pixels in a defined region of interest, were obtained automatically. Receiver operating characteristic curve analysis was performed to verify the diagnostic performance of MVUS. The interclass correlation coefficient and Cohen’s kappa analysis were used to analyze the reliability of MVUS (ClinicalTrials.gov:NCT04879173).
Results
The area under the curve (AUC) for CD, PD, MVUS, and MVUS-VI was 0.822, 0.844, 0.808, and 0.852 respectively. The optimal cutoff value of the MVUS-VI was 24.95% for distinguishing GD and DT with 87% sensitivity and 80.9% specificity. We found a significant positive correlation of MVUS-VI with thyrotropin receptor antibody (r=0.554) and with thyroid stimulating immunoglobulin bioassay (r=0.841). MVUS showed high intra- and inter-observer reliability from various statistical method.
Conclusion
In a real time and quantitative manner, MVUS-VI could be helpful to differentiate GD from thyroiditis in thyrotoxic patients, with less inter-observer variability. -
Citations
Citations to this article as recorded by- Association of autoimmune thyroid disease with type 1 diabetes mellitus and its ultrasonic diagnosis and management
Jin Wang, Ke Wan, Xin Chang, Rui-Feng Mao
World Journal of Diabetes.2024; 15(3): 348. CrossRef - The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
Endocrinology and Metabolism.2023; 38(3): 338. CrossRef - Duplex Hemodynamic Parameters of Both Superior and Inferior Thyroid Arteries in Evaluation of Thyroid Hyperfunction Disorders
Maha Assem Hussein, Alaa Abdel Hamid, Rasha M Abdel Samie, Elshaymaa Hussein, Shereen Sadik Elsawy
International Journal of General Medicine.2022; Volume 15: 7131. CrossRef - Case 5: A 41-Year-Old Woman With Palpitation
Jiwon Yang, Kabsoo Shin, Jeongmin Lee, Jeonghoon Ha, Dong-Jun Lim, Han-Sang Baek
Journal of Korean Medical Science.2022;[Epub] CrossRef - Microvascular assessment of fascio-cutaneous flaps by ultrasound: A large animal study
Guillaume Goudot, Yanis Berkane, Eloi de Clermont-Tonnerre, Claire Guinier, Irina Filz von Reiterdank, Antonia van Kampen, Korkut Uygun, Curtis L. Cetrulo, Basak E. Uygun, Anahita Dua, Alexandre G. Lellouch
Frontiers in Physiology.2022;[Epub] CrossRef
- Association of autoimmune thyroid disease with type 1 diabetes mellitus and its ultrasonic diagnosis and management

- Calcium & Bone Metabolism
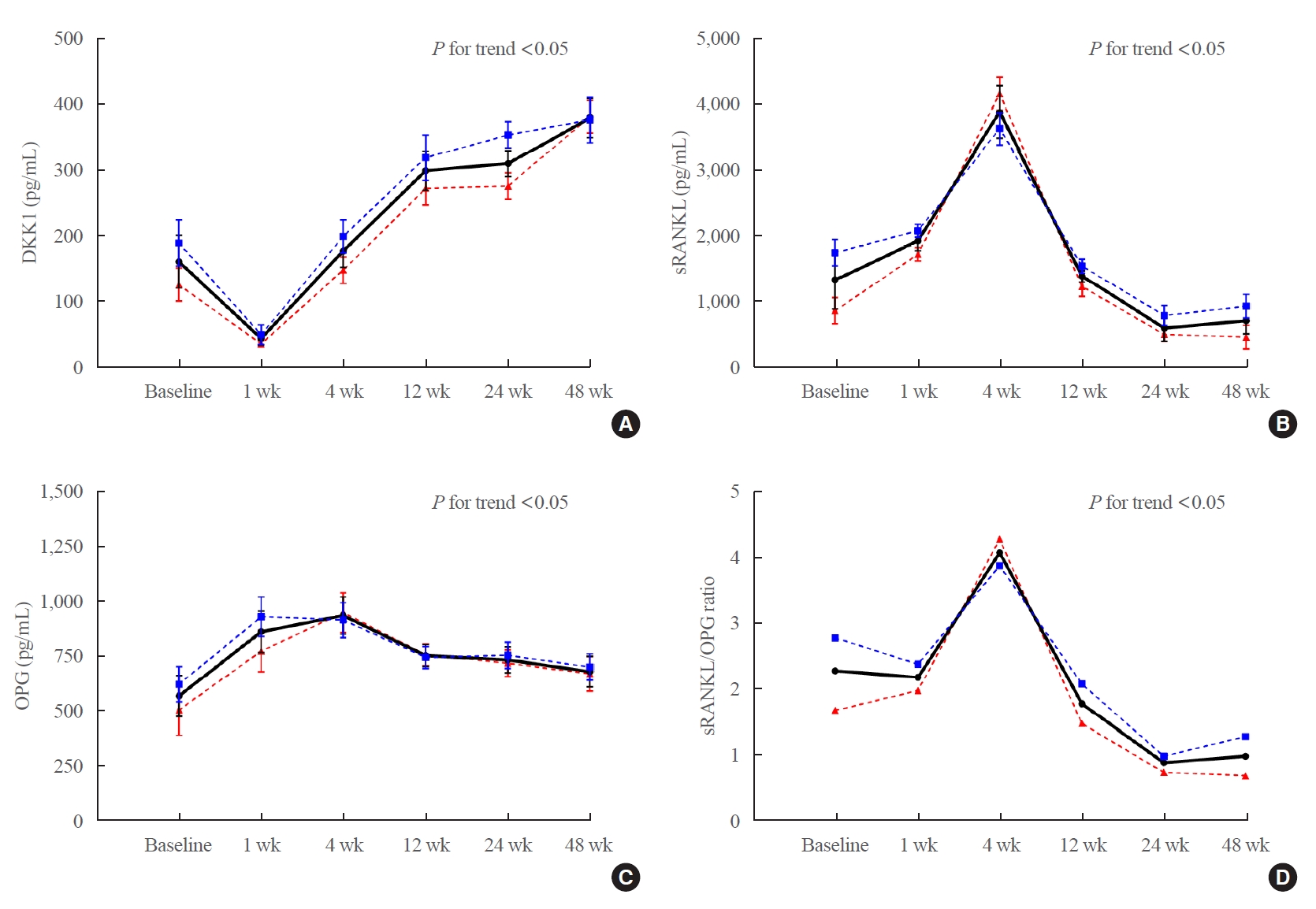
- Changes in Serum Dickkopf-1, RANK Ligand, Osteoprotegerin, and Bone Mineral Density after Allogeneic Hematopoietic Stem Cell Transplantation Treatment
- Eunhee Jang, Jeonghoon Ha, Ki-Hyun Baek, Moo Il Kang
- Endocrinol Metab. 2021;36(6):1211-1218. Published online December 8, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1248
- 2,973 View
- 104 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Dickkopf-1 (DKK1) regulates bone formation by inhibiting canonical Wnt/β-catenin pathway signaling, and indirectly enhances osteoclastic activity by altering the expression ratio of receptor activator of nuclear factor-κB ligand (RANKL) relative to osteoprotegerin (OPG). However, it is difficult to explain continued bone loss after allogeneic stem cell transplantation (allo-SCT) in terms of changes in only RANKL and OPG. Few studies have evaluated changes in DKK1 after allo-SCT.
Methods
We prospectively enrolled 36 patients with hematologic malignancies who were scheduled for allo-SCT treatment. Serum DKK1, OPG, and RANKL levels were measured before (baseline), and at 1, 4, 12, 24, and 48 weeks after allo-SCT treatment. Bone mineral density (BMD) was assessed using dual-energy X-ray absorptiometry before (baseline) and 24 and 48 weeks after allo-SCT treatment.
Results
After allo-SCT treatment, the DKK1 level decreased rapidly, returned to baseline during the first 4 weeks, and remained elevated for 48 weeks (P<0.0001 for changes observed over time). The serum RANKL/OPG ratio peaked at 4 weeks and then declined (P<0.001 for changes observed over time). BMD decreased relative to the baseline at all timepoints during the study period, and the lumbar spine in female patients had the largest decline (–11.3%±1.6% relative to the baseline at 48 weeks, P<0.05).
Conclusion
Serum DKK1 levels rapidly decreased at 1 week and then continued to increase for 48 weeks; bone mass decreased for 48 weeks following engraftment in patients treated with allo-SCT, suggesting that DKK1-mediated inhibition of osteoblast differentiation plays a role in bone loss in patients undergoing allo-SCT. -
Citations
Citations to this article as recorded by- Fracture risk and assessment in adults with cancer
Carrie Ye, William D. Leslie
Osteoporosis International.2023; 34(3): 449. CrossRef - Short-Term Impact of Hematopoietic Stem Cell Transplantation in Leukemia Patients on Bone Bio Markers, Electrolytes and Blood Profile
Rhythm Joshi, Zehva Khan, Aakriti Garg, Dinesh Bhurani, Nidhi B Agarwal, Ubada Aqeel, Mohd Ashif Khan
OBM Transplantation.2023; 07(02): 1. CrossRef
- Fracture risk and assessment in adults with cancer

- Thyroid
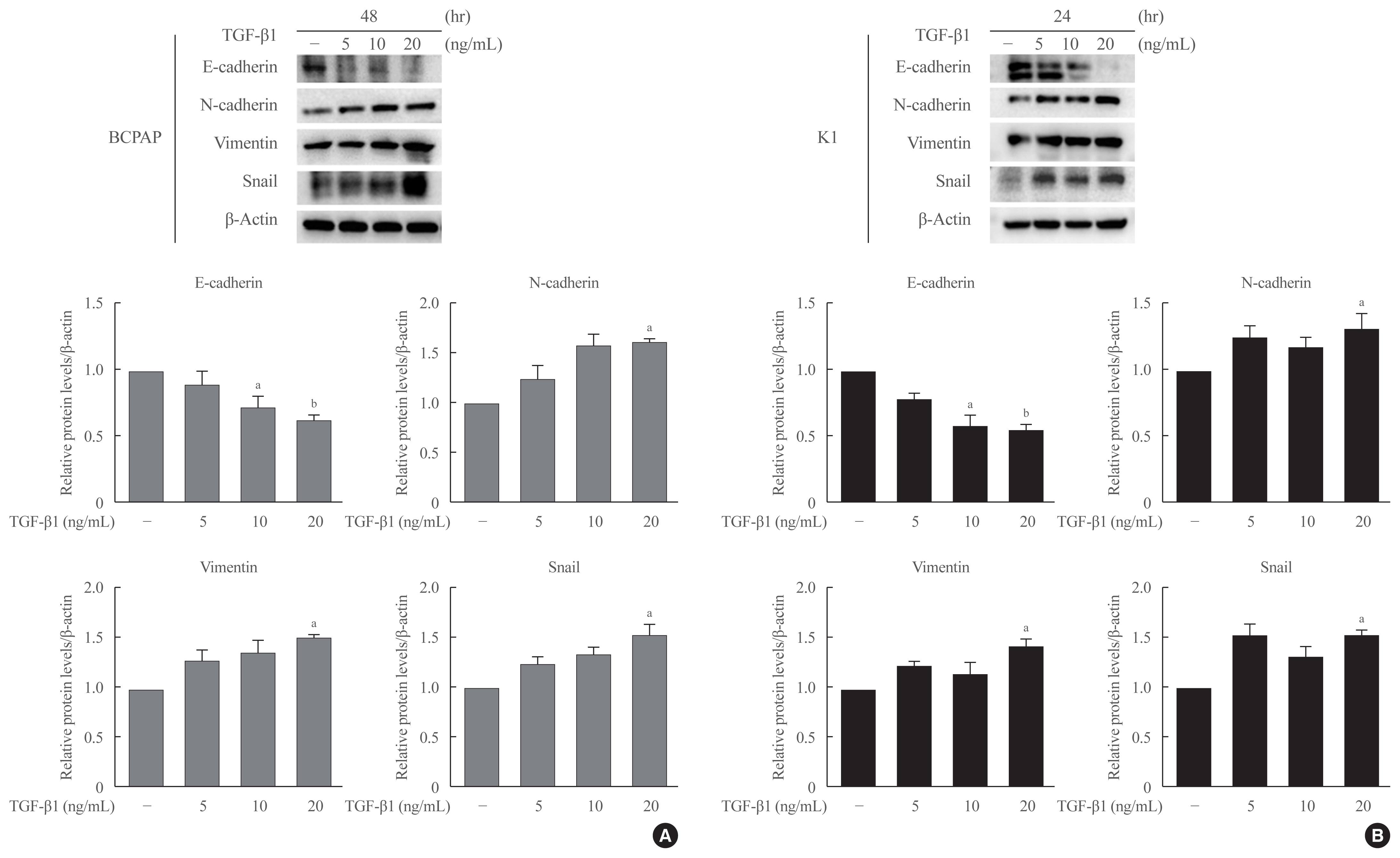
- Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
- Jun-Qing Jin, Jeong-Sun Han, Jeonghoon Ha, Han-Sang Baek, Dong-Jun Lim
- Endocrinol Metab. 2021;36(5):1095-1110. Published online October 14, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1155
- 4,743 View
- 159 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) ligands have been widely shown to correlate with epithelial-mesenchymal transition (EMT) and cancer progression. Lobeglitazone (LGZ) is a novel ligand of PPAR-γ; and its role in EMT and metastasis in papillary thyroid carcinoma (PTC) is poorly understood. We aimed to investigate the role of LGZ in metastatic behavior of PTC cells.
Methods
Half maximal inhibitory concentration (IC50) values of LGZ in BRAF-mutated PTC cell lines (BCPAP and K1) were determined using MTT assay. Rosiglitazone (RGZ), the PPAR-γ ligand was used as a positive control. The protein expression of PPAR-γ, cell-surface proteins (E-cadherin, N-cadherin), cytoskeletal protein (Vimentin), transcription factor (Snail), p38 mitogenactivated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) 1/2 pathway, and matrix metalloproteinase (MMP)-2 expression were measured using Western blotting. Changes in E-cadherin expression were also determined using immunocytochemistry. Cell migration and invasion were analyzed using wound healing and Matrigel invasion assays.
Results
Treatment with LGZ or RGZ significantly inhibited transforming growth factor-beta1 (TGF-β1)-induced EMT-associated processes such as fibroblast-like morphological changes, EMT-related protein expression, and increased cell migration and invasion in BCPAP and K1 cells. LGZ restored TGF-β1-induced loss of E-cadherin, as observed using immunocytochemistry. Furthermore, LGZ and RGZ suppressed TGF-β1-induced MMP-2 expression and phosphorylation of p38 MAPK, but not ERK1/2. Although there was no change in PPAR-γ expression after treatment with LGZ or RGZ, the effect of downstream processes mediated by LGZ was hampered by GW9662, a PPAR-γ antagonist.
Conclusion
LGZ inhibits TGF-β1-induced EMT, migration, and invasion through the p38 MAPK signaling pathway in a PPAR-γ-dependent manner in PTC cells. -
Citations
Citations to this article as recorded by- Diabetes Mellitus and Thyroid Cancers: Risky Correlation, Underlying Mechanisms and Clinical Prevention
Rongqian Wu, Junping Zhang, Guilin Zou, Shanshan Li, Jinying Wang, Xiaoxinlei Li, Jixiong Xu
Diabetes, Metabolic Syndrome and Obesity.2024; Volume 17: 809. CrossRef - Clinicopathological Evaluation of Papillary Thyroid Microcarcinoma
Ando Takahito, Kimihito Fujii, Hirona Banno, Masayuki Saito, Yukie Ito, Mirai Ido, Manami Goto, Yukako Mouri, Junko Kousaka, Tsuneo Imai, Shogo Nakano
Cureus.2024;[Epub] CrossRef - Pioglitazone, a peroxisome proliferator‑activated receptor γ agonist, induces cell death and inhibits the proliferation of hypoxic HepG2 cells by promoting excessive production of reactive oxygen species
Guohao Huang, Mengfan Zhang, Manzhou Wang, Wenze Xu, Xuhua Duan, Xinwei Han, Jianzhuang Ren
Oncology Letters.2024;[Epub] CrossRef - The Activation of PPARγ by (2Z,4E,6E)-2-methoxyocta-2,4,6-trienoic Acid Counteracts the Epithelial–Mesenchymal Transition Process in Skin Carcinogenesis
Enrica Flori, Sarah Mosca, Giorgia Cardinali, Stefania Briganti, Monica Ottaviani, Daniela Kovacs, Isabella Manni, Mauro Truglio, Arianna Mastrofrancesco, Marco Zaccarini, Carlo Cota, Giulia Piaggio, Mauro Picardo
Cells.2023; 12(7): 1007. CrossRef - Cumulative exposure to metabolic syndrome increases thyroid cancer risk in young adults: a population-based cohort study
Jinyoung Kim, Kyungdo Han, Mee Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
The Korean Journal of Internal Medicine.2023; 38(4): 526. CrossRef - Drug repositioning in thyroid cancer treatment: the intriguing case of anti-diabetic drugs
Alessia Greco, Francesca Coperchini, Laura Croce, Flavia Magri, Marsida Teliti, Mario Rotondi
Frontiers in Pharmacology.2023;[Epub] CrossRef - Fish and the Thyroid: A Janus Bifrons Relationship Caused by Pollutants and the Omega-3 Polyunsaturated Fatty Acids
Salvatore Benvenga, Fausto Famà, Laura Giovanna Perdichizzi, Alessandro Antonelli, Gabriela Brenta, Francesco Vermiglio, Mariacarla Moleti
Frontiers in Endocrinology.2022;[Epub] CrossRef - Identifying and categorizing compounds that reduce corneal transforming growth factor beta induced protein levels: a scoping review
Gabriella Guo Sciriha, Janet Sultana, Joseph Borg
Expert Review of Clinical Pharmacology.2022; 15(12): 1423. CrossRef
- Diabetes Mellitus and Thyroid Cancers: Risky Correlation, Underlying Mechanisms and Clinical Prevention

Special Article
- Miscellaneous
- COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society
- Cheol Ryong Ku, Kyong Yeun Jung, Chang Ho Ahn, Jun Sung Moon, Ju Hee Lee, Eun Heui Kim, Hyemi Kwon, Hee Kyung Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Eun Roh, Jin Hwa Kim, Mi-kyung Kim, the Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2021;36(4):757-765. Published online August 17, 2021
- DOI: https://doi.org/10.3803/EnM.2021.404
- 10,360 View
- 419 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Since the first outbreak of coronavirus disease 2019 (COVID-19), ongoing efforts have been made to discover an efficacious vaccine against COVID-19 to combat the pandemic. In most countries, both mRNA and DNA vaccines have been administered, and their side effects have also been reported. The clinical course of COVID-19 and the effects of vaccination against COVID-19 are both influenced by patients’ health status and involve a systemic physiological response. In view of the systemic function of endocrine hormones, endocrine disorders themselves and the therapeutics used to treat them can influence the outcomes of vaccination for COVID-19. However, there are very limited data to support the development of clinical guidelines for patients with specific medical backgrounds based on large clinical trials. In the current severe circumstances of the COVID-19 pandemic, position statements made by clinical specialists are essential to provide appropriate recommendations based on both medical evidence and clinical experiences. As endocrinologists, we would like to present the medical background of COVID-19 vaccination, as well as precautions to prevent the side effects of COVID-19 vaccination in patients with specific endocrine disorders, including adrenal insufficiency, diabetes mellitus, osteoporosis, autoimmune thyroid disease, hypogonadism, and pituitary disorders.
-
Citations
Citations to this article as recorded by- COVID-19 mRNA vaccine may trigger subacute thyroiditis
Mehmet Sözen, Ömercan Topaloğlu, Berrin Çetinarslan, Alev Selek, Zeynep Cantürk, Emre Gezer, Damla Köksalan, Taner Bayraktaroğlu
Human Vaccines & Immunotherapeutics.2024; 17(12): 5120. CrossRef - The role of co-morbidities in the development of an AEFI after COVID-19 vaccination in a large prospective cohort with patient-reported outcomes in the Netherlands
C. Ouaddouh, J.W. Duijster, T. Lieber, F.P.A.M. van Hunsel
Expert Opinion on Drug Safety.2024; 23(3): 323. CrossRef - Thyroid dysfunction in COVID-19
David Tak Wai Lui, Chi Ho Lee, Yu Cho Woo, Ivan Fan Ngai Hung, Karen Siu Ling Lam
Nature Reviews Endocrinology.2024;[Epub] CrossRef - Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
Hyeyeon Moon, Sunghwan Suh, Mi Kyoung Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Prior immunization status of COVID-19 patients and disease severity: A multicenter retrospective cohort study assessing the different types of immunity
Javaria Aslam, Faisal Shahzad Khan, Muhammad Talha Haris, Hewad Hewadmal, Maryam Khalid, Mohammad Y. Alshahrani, Qurrat-ul-ain Aslam, Irrum Aneela, Urooj Zafar
Vaccine.2023; 41(2): 598. CrossRef - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(2): 253. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Inactivated SARS-CoV-2 vaccination does not disturb the clinical course of Graves’ disease: An observational cohort study
Shichen Xu, Huixin Yu, Xian Cheng, Jing Wu, Jiandong Bao, Li Zhang
Vaccine.2023; 41(38): 5648. CrossRef - Adrenal Crisis Associated With COVID-19 Vaccination in Patients With Adrenal Insufficiency
Yukako Kurematsu, Takako Mohri, Sadanori Okada, Yutaka Takahashi
JCEM Case Reports.2023;[Epub] CrossRef - Adverse Events Associated with COVID-19 Vaccination in Adolescents with Endocrinological Disorders: A Cross-Sectional Study
İbrahim Mert Erbaş, İrem Ceren Erbaş, Gözde Akın Kağızmanlı, Kübra Yüksek Acinikli, Özge Besci, Korcan Demir, Ece Böber, Nurşen Belet, Ayhan Abacı
Journal of Clinical Research in Pediatric Endocrinology.2023; 15(3): 248. CrossRef - Neue Aspekte der Glukokortikoidsubstitution bei Nebennierenrindeninsuffizienz
Tina Kienitz, Gesine Meyer
Der Internist.2022; 63(1): 12. CrossRef - Endocrine Follow-up During Post-Acute COVID-19: Practical Recommendations Based on Available Clinical Evidence
Rimesh Pal, Ameya Joshi, Sanjay K. Bhadada, Mainak Banerjee, Suresh Vaikkakara, Satinath Mukhopadhyay
Endocrine Practice.2022; 28(4): 425. CrossRef - Safety of Inactivated and mRNA COVID-19 Vaccination Among Patients Treated for Hypothyroidism: A Population-Based Cohort Study
Xi Xiong, Carlos King Ho Wong, Ivan Chi Ho Au, Francisco Tsz Tsun Lai, Xue Li, Eric Yuk Fai Wan, Celine Sze Ling Chui, Esther Wai Yin Chan, Franco Wing Tak Cheng, Kristy Tsz Kwan Lau, Chi Ho Lee, Yu Cho Woo, David Tak Wai Lui, Ian Chi Kei Wong
Thyroid.2022; 32(5): 505. CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Adrenal Crisis Secondary to COVID-19 Vaccination in a Patient With Hypopituitarism
Nikolina Markovic, Anila Faizan, Chirag Boradia, Sridhar Nambi
AACE Clinical Case Reports.2022; 8(4): 171. CrossRef - The Effect of Inactivated SARS-CoV-2 Vaccines on TRAB in Graves’ Disease
LingHong Huang, ZhengRong Jiang, JingXiong Zhou, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Osteoporosis in Patients With Respiratory Diseases
Yue Ma, Shui Qiu, Renyi Zhou
Frontiers in Physiology.2022;[Epub] CrossRef - Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology
Ach Taieb, El Euch Mounira
Vaccines.2022; 10(12): 2004. CrossRef - Forty Years Together, New Leap Forward! The 40th Anniversary of the Korean Endocrine Society
Jong Chul Won, Ki-Hyun Baek
Endocrinology and Metabolism.2022; 37(6): 851. CrossRef - No need of glucocorticoid dose adjustment in patients with adrenal insufficiency before COVID-19 vaccine
Tania Pilli, Cristina Dalmiglio, Gilda Dalmazio, Alfonso Sagnella, Raffaella Forleo, Lucia Brilli, Fabio Maino, Cristina Ciuoli, Maria Grazia Castagna
European Journal of Endocrinology.2022; 187(1): K7. CrossRef - Diabetes and COVID-19 Vaccination
Hae Dong Choi, Jun Sung Moon
The Journal of Korean Diabetes.2021; 22(4): 221. CrossRef
- COVID-19 mRNA vaccine may trigger subacute thyroiditis

Original Article
- Bone Metabolism
- Comparison of the Effects of Various Antidiabetic Medication on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus
- Jeonghoon Ha, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Seung Hyun Ko, Moo Il Kang, Sung Dae Moon, Ki-Hyun Baek
- Endocrinol Metab. 2021;36(4):895-903. Published online August 9, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1026

- 6,099 View
- 230 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Prospective comparative studies on the effects of various antidiabetic agents on bone metabolism are limited. This study aimed to assess changes in bone mass and biochemical bone markers in postmenopausal patients with type 2 diabetes mellitus (T2DM).
Methods
This prospective, multicenter, open-label, comparative trial included 264 patients with T2DM. Patients who had received a metformin, or sulfonylurea/metformin combination (Group 1); a thiazolidinedione combination (Group 2); a dipeptidyl peptidase-4 inhibitor (gemigliptin) combination (Group 3); or an sodium-glucose cotransporter 2 inhibitor (empagliflozin) combination (Group 4) were prospectively treated for 12 months; bone mineral density (BMD) and bone turnover marker (BTM) changes were evaluated.
Results
The femoral neck BMD percentage changes were −0.79%±2.86% (Group 1), −2.50%±3.08% (Group 2), −1.05%±2.74% (Group 3), and −1.24%±2.91% (Group 4) (P<0.05). The total hip BMD percentage changes were −0.57%±1.79% (Group 1), −1.74%±1.48% (Group 2), −0.75%±1.87% (Group 3), and −1.27%±1.72% (Group 4) (P<0.05). Mean serum BTM (C-terminal type 1 collagen telopeptide and procollagen type 1 amino-terminal propeptide) levels measured during the study period did not change over time or differ between groups.
Conclusion
Significant bone loss in the femoral neck and total hip was associated with thiazolidinedione combination regimens. However, bone loss was not significantly associated with combination regimens including gemigliptin or empagliflozin. Caution should be exercised during treatment with antidiabetic medications that adversely affect the bone in patients with diabetes at a high risk of bone loss. -
Citations
Citations to this article as recorded by- Meta-Analysis on the Association Between DPP-4 Inhibitors and Bone Mineral Density and Osteoporosis
Lili Huang, Wei Zhong, Xinghuan Liang, Huijuan Wang, Shi-en Fu, Zuojie Luo
Journal of Clinical Densitometry.2024; 27(1): 101455. CrossRef - A multicentre, double‐blind, placebo‐controlled, randomized, parallel comparison, phase 3 trial to evaluate the efficacy and safety of pioglitazone add‐on therapy in type 2 diabetic patients treated with metformin and dapagliflozin
Soo Lim, Seung‐Hwan Lee, Kyung‐Wan Min, Chang Beom Lee, Sang Yong Kim, Hye Jin Yoo, Nan Hee Kim, Jae Hyeon Kim, Seungjoon Oh, Jong Chul Won, Hyuk Sang Kwon, Mi Kyung Kim, Jung Hwan Park, In‐Kyung Jeong, Sungrae Kim
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Association of Bone Turnover Markers with Type 2 Diabetes Mellitus and Microvascular Complications: A Matched Case-Control Study
Yilin Hou, Xiaoyu Hou, Qian Nie, Qiuyang Xia, Rui Hu, Xiaoyue Yang, Guangyao Song, Luping Ren
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 1177. CrossRef - Complementary effects of dapagliflozin and lobeglitazone on metabolism in a diet-induced obese mouse model
Yun Kyung Lee, Tae Jung Oh, Ji In Lee, Bo Yoon Choi, Hyen Chung Cho, Hak Chul Jang, Sung Hee Choi
European Journal of Pharmacology.2023; 957: 175946. CrossRef
- Meta-Analysis on the Association Between DPP-4 Inhibitors and Bone Mineral Density and Osteoporosis


 KES
KES

 First
First Prev
Prev



